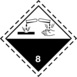
Potassium hydroxide, flakes, KOH One of the simplest inorganic compounds belonging to the group of hydroxides. It is one of the strongest bases of pH 14. Under normal conditions – at room temperature and atmospheric pressure – it has the form of white brittle flakes. The product tends to clump.
Potassium hydroxide dissolves well in water, but generates significant amounts of heat. Also available on the market in the form of aqueous solutions, i.e. potassium lye. Potassium hydroxide is a highly hygroscopic substance. It naturally absorbs moisture and carbon dioxide from the air. Then it spontaneously transforms into potassium carbonate. Therefore, it is recommended to store it in airtight packaging and in warehouses with low air humidity.
On an industrial scale, it is obtained in the process of electrolysis of an aqueous solution of potassium chloride.
Potassium hydroxide is used in many industries, including: cosmetics, textiles, printing, paper, plastics, furniture, and in chemical synthesis processes as a reagent.
The automatically translated description is binding in the Polish version.